The use of a continuous passive motion (CPM) machine was initiated around 1982 after total knee replacements (TKA) to promote healing to the tissues, but other benefits found from CPM included increased ROM, decrease pain and the need for analgesics, and decreased rate of deep vein thrombosis (DVT). [6, 12, 21, 22] Ritter et al [20] however, found that the use of the CPM lead to a weaker leg, flexion tightness and extension lag along with increased cost with its use. There have been multiple studies performed over the past several years looking at the use of CPM and its efficacy and effectiveness following TKA, and the results were variable and contradictory. [1,3,5,13,14,17,18,29] Many of these studies looked at the benefits on range of motion short-term and/or long-term as the primary benefit of using CPM and most of the studies concluded that the CPM did not change the long-term outcomes of ROM considering the increased cost of the device. [18, 29] The post-operative rehabilitation protocols of using CPM also varied from study to study, which makes it difficult to conclude that the use of CPM was not effective. There were few studies that looked at the cost benefits and the benefits of reducing DVT and other complications [16, 18, 29]. These studies found that the use of CPM did not have a negative effect on the incidence of DVT, other complications, or the number of days in the hospital. McInnes et al [18] looked at the efficacy of CPM use on outcomes of range of motion, pain, swelling, quadriceps strength, length of stay and complications following a TKA. These studies also vary in their post-operative protocols and patient’s time using CPM also varied within the studies. There were no studies found that utilized the CPM for any other post-operative knee treatment other than TKA procedures.
CLINICAL QUESTION
In our facility, we have been using CPM for all of our post-operatively as part of a total pain management and rehabilitation program that prevents a hemarthrosis. We have been using CPM since 1984 for our patients after anterior cruciate ligament (ACL) reconstruction. The lessons learned from our analysis of data collected over the past 37 years on our ACL patients were implemented with our TKA patients in 2007. With the increasing Opioid problem in our country, the predicted rise in number of TKA procedures performed, and the reporting of some poor outcomes following ACL reconstruction, we still have the clinical question of whether or not the use of CPM post-operatively have any benefits? We propose that the use of a CPM post-operatively as on part of a pain management program is beneficial.
POST-OPERATIVE PROTOCOL:
Our post-operative protocol includes a combination of modalities, physical therapy exercises, and pain medications that are used for the primary purpose of preventing a hemarthrosis in the knee, which is the main culprit that causes pain and limitations in knee range of motion and leg function after surgery. The primary advantage of using a CPM post-operatively is that it allows the knee to stay above the heart while patients are on bedrest. This prevents a hemarthrosis in the knee joint, which has secondary effects on improved range of motion, decreased pain and no quadriceps inhibition. The CPM moves the knee from 0 degrees to 30 degrees, 24 hours a day, while on bedrest. Additionally, a mid-thigh TED hose stocking is applied over post-operative dressings in the operating room at the end of the procedure, along with a cold/compression device. The elastic stocking compress the superficial veins in the lower limbs. It is used in post-operative patients and others immobilized by illness to prevent thrombophlebitis by shunting blood through the deep veins of the calves and thighs. The patient, and their caregiver, is instructed, before surgery, on how to operate the cold/compression device and are instructed to change the water out of the device every hour during the first week to allow for constant compression to the joint capsule, and cold/compression at least 15 minutes out of every hour of the day. The device is removed from the knee when the patient gets up to go to the bathroom, and it comes off when doing a series of exercises three times a day. This allows for a more normal gait pattern and avoids onset of compensatory patterns in gait. Patients may use an assistive device as needed for safety. Otherwise, the patient is educated on staying on bedrest with the leg elevated above the heart in the CPM during the first 7 days post-operatively.
Patients are instructed in a series of exercises to be done 3 times/day and log sheets are provided to make sure exercises are completed and patients can document range of motion as measured with a yardstick. The exercises include taking the CPM to 120 degrees and holding for 1 minute for ACL patients, and 3 minutes for the TKA patients. They are given a yardstick and are told to use the centimeter side to measure and record a heel slide, with the measurement to be recorded where the heel lands on the yardstick. The third exercise is doing a heel prop on a firm bolster so that the knee is suspended for 5 minutes to facilitate achieving full extension including hyperextension. The fourth exercise is doing five repetitions of straight leg raise to ensure that there is no loss of quadriceps muscle control during the first week and the patient is educated to independently lift the leg, not help the lower extremity. The last exercise is the towel stretch with an active heel lift to ensure full extension and quadriceps control following surgery.
PAIN MANAGEMENT PROTOCOL
Another component with our post-operative protocol is preemptive pain management, which is defined as prevention of pain prior to surgical insult. Our current protocol evolved since the 1980 is starting with the use of IM Demerol to using IM Ketorolac every 6 hours, and eventually to using continuous infusion IV Ketorolac, which is our current pain management approach. Ketorolac is a preemptive pain management drug that prevents prostaglandin synthesis when it is administered before surgery, causing a decrease in local inflammatory reaction. A maximum dose of Ketorolac of 120mg/day is allowed. There were no kidney or liver complications noted, and it allowed for smoother pain control than using injections. The current IV Ketorolac pain management protocol is as follows:
- 30mg IV push pre-op before the tourniquet is inflated
- The remaining 90mg is infused in a 1000mL bag of normal saline set to run at 40mL/hour until the dose was finished.
- The dose can be adjusted to 60mL/hour for 15 minutes to provide adequate pain control with the maximum dose still kept at 120mg/day.
- Acetaminophen 1000 mg every 6 hours beginning when the patient tolerates oral medication.
- Tramadol, 50 mg, every 4-6 hours if needed.
- The following day, the physician gives 60 mg bolus of Ketorolac in the IV before it is removed to prevent patients from having a rebound of pain when they travel home.
- 440 mg of Naproxen administered every 12 hours beginning after the Ketorolac drip is finished.
KEY RESULTS
This approach to managing and preventing a post-operative hemarthrosis has been used at our facility for ACL reconstruction patients since 1984. Shelbourne and Nitz [25] published the results of using this protocol with an “accelerated rehabilitation program after ACL reconstruction”, and the results indicated a quicker return of ROM and strength, with no loss of stability and fewer complications, which inadvertently lead to quicker return to sport. Other subsequent studies have gone on to find that achieving normal knee range of motion post-operatively is vital for achieving not only the best subjective outcomes but for reducing the risk for developing osteoarthritis in the long-term after surgery. [26, 27, 28]
We have continually analyzed our results over past 37 years, and have made a few modifications in our post-operative rehabilitation protocol in order to continue to make improvements in our patient’s overall outcomes. We have also tracked pain levels, need for pain medication and long-term outcomes with ROM, strength and function with not only the ACL patient populations, but with our total knee arthroplasty procedures, beginning in 2007. As we have collected data from the TKA patients, we are finding the same benefit with no hemarthrosis, decrease pain levels, decreased need of pain medication, increased return of range of motion and no quad inhibition. Patients are walking without the need of any assistive device sooner.
PAIN MANAGEMENT STUDY WITH ACL PATIENTS
Shelbourne, et al did a retrospective study that compared results between three different pain control protocols. [24] Group 1 (1987-1991; 707 patients) received IM Demerol injections. Group 2 (1991-1994; 498 patients) received IM Ketorolac injections, scheduled every 6 hours. Group 3 (1994-1995; 313 patients) received continuous infusion IV Ketorolac. Group 3 had a statistically significantly lower mean narcotic doses/day and per/hour, shorter hospital stay, and lower hemovac output (P<.05; (Table 1).
Table 1. Comparison of narcotic pain medication use between ACL reconstruction patients.
| IM Demerol Group | IM ketorolac Group | IV ketorolac Group | |
| Mean narcotic dose per day | 3.7 | 2.5 | 1.9 |
| Mean hemovac output (mL) | 246 | 230 | 225 |
| Mean hospital stay (hours) | 76.7 | 54.5 | 37.6 |
ACL – Anterior cruciate ligament
IM – Intramuscular
IV – Intravenous
PAIN MANAGEMENT STUDY WITH TKA PATIENTS
Because of the effectiveness of the pain protocol with ACL patients, we thought it should be effective with our TKA patients. However, the practice of bedrest and use of ketorolac raised concerns because this was not considered as standard of practice for this population. These concerns included keeping elderly patients on bedrest might cause an increase in DVT. Another concern was the use of ketorolac-continuous infusion for 5 days was found to cause GI ulcerations in 1-10% of patients, acute coronary syndromes or impaired renal function in <1%, lack of bone healing found in animal lab studies, and some patients developed transient increased creatine levels. [8, 9] However, none of the other studies used any of the other parts of our protocol that would mitigate these types of complications. To determine whether our protocol caused increased complications, a study was conducted to retrospectively analyze our TKA data of pain medication use, pain level and complication rate vs. another group of patients who were mobilized every day and who had an Opioid pain control. (Table 2) [23] All patients received peri-articular injection with bupivacaine 0.25% and epinephrine.
Table 2. Comparison of continuous infusion ketorolac versus opioid protocols.
| Ketorolac Group | Opioid Group | |
| Preoperative medication | Ketorolac 30 mg IV bolus intraoperatively before inflation of the tourniquet | None |
| Postoperative medication |
|
|
| Rehabilitation | Bedrest for the 7 days with TED hose, cold/compression, leg elevated above heart in the CPM 0-30 degrees, exercise session 3x/day and up only for bathroom privileges. | Standardized TKA rehabilitation program that encouraged early ambulation on day of surgery. |
The results of this study showed that patients in the ketorolac/bedrest protocol had statistically significantly lower pain scores and less narcotic use than the opioid/early ambulation protocol. Patients in the CI ketorolac group receiving 81% fewer opioids in the first 24 hours and 95% less in the second 24 hours. (Table 3)
Table 3. Morphine equivalent units (MEU) between TKA patient groups:
| Time (hours) after surgery | Ketorolac group MEU | Opioid group | P-value |
| 0-24 | 26.3 ± 31.8 | 136 ± 33.1 | <.001 |
| 24-38 | 7.7 ± 12.6 | 165 ± 9.1 | <.001 |
The ketorolac group also had a statistically significant lower percentage of those suffering with respiratory depression at 5.2% versus 25.3% in the opioid group. Respiratory depression is defined as less than 8 breaths/minute or Naloxone administered. There were six patients in the Opioid group for whom Naloxone was administered and none in the ketorolac group.
CURRENT RESTULTS IN OUR TKA PATIENTS
Strict bedrest has been the standard of care treatment for patients with DVTs; however, there has also been concern that keeping older patients on bedrest following a TKA will lead to DVT. There are several studies that discuss the advantage of early ambulation following joint replacement with no associated increased incidence of acute DVT following TKA when anticoagulation management was also utilized [4,7,10,11,15,19] However, for 1119 patients who underwent a TKA surgery and followed our post-operative protocol between 2007 and 2018, 3 patients suffered a DVT (0.27%). Therefore, our treatment approach of keeping the patient on bedrest and elevating in a CPM does not lead to increase rate of DVT.
Because our philosophy has always put range of motion first with our rehabilitation programs, we feel that there should not be any limitations for achieving full, symmetrical range of motion with our ACL patients and feel there should not be any limitations to our TKA population as well. Of 1029 TKAs in 841 patients, there was a mean age of 65.0 with 619 unilateral patients, 157 bilateral patients, and 96 staged procedures and we were able to collect 1 year objective data, or greater on 60%. The post-operative complications included 11 (1.2%) manipulation for flexion loss, 10 (1.1%) infection, and 3 quad tendon ruptures. Pre-operative knee extension showed 62% had 0° or some degree of hyperextension. However, 90% had 0° of extension at 1 year and a quarterly report shows that 94% have at least 0° at 6 months post-operative and 84% at 3 months. With flexion range of motion, the mean flexion range of motion is 121° pre-operatively and the mean 123° at 2 years post-operatively.
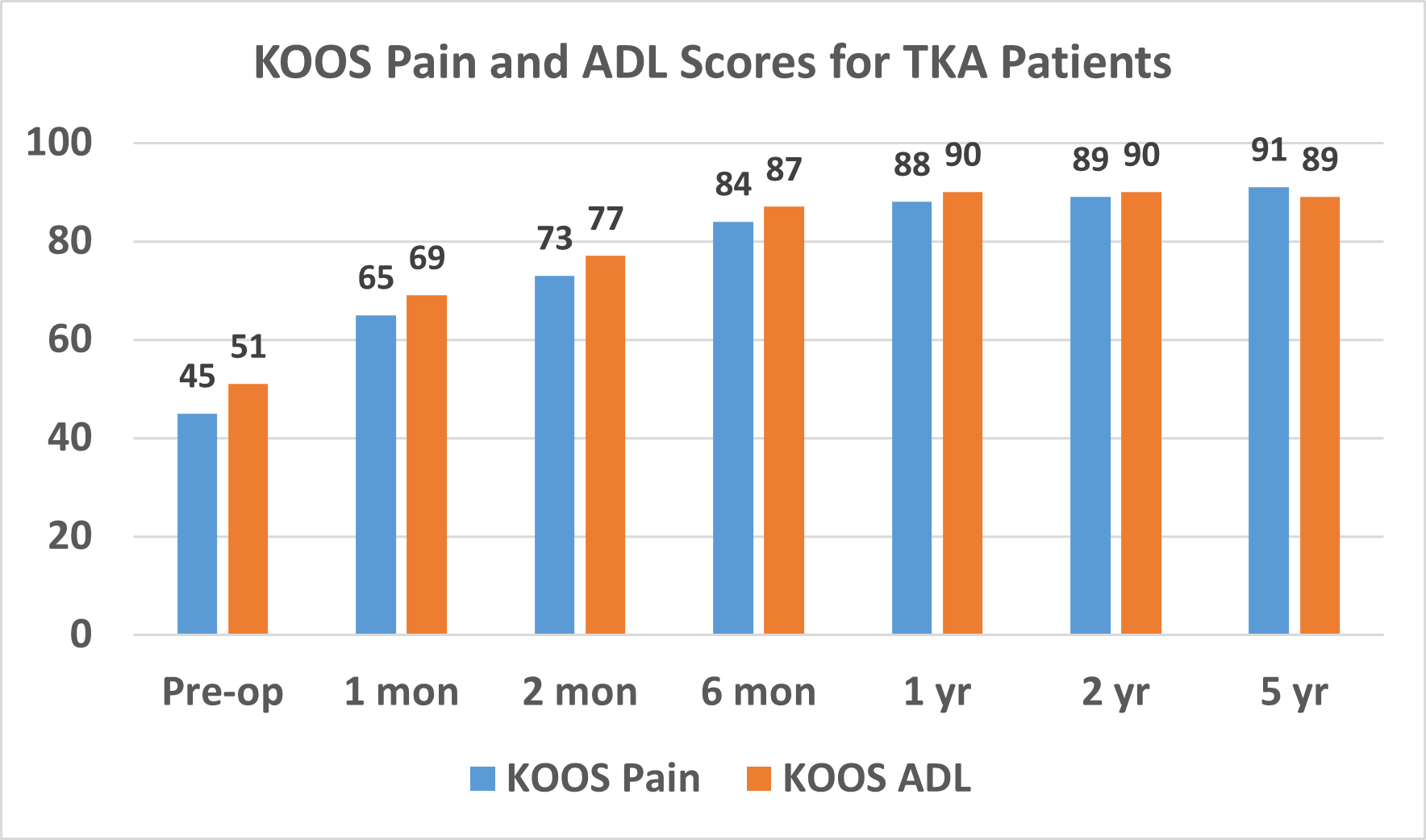
Patients routinely complete KOOS surveys to help surveys to help us monitor our results. The mean KOOS Pain and ADL scores pre-operatively and through 5 years post-operatively are shown in Figure 1. The percentage of patients who had KOOS sub-score values equal to a normative population was 93% for pain, 84% for symptoms, 96% for ADL, 90% for sport, and 91% for quality of life.
Figure 1. Mean KOOS Pain and Activities of Daily Living (ADL) scores for TKA patients.
DISCUSSION
Effective pain management in the first week using the TED hose, staying on bedrest with leg elevated in the CPM 24 hours a day for 7 days, using a cold/compression device, and doing exercises 3x/day in bed allows us to be aggressive with achieving full range of motion quickly after surgery. A major component to our perioperative rehabilitation program with both our ACL and TKA patients is the time we spend with patients pre-operatively to achieve the best range of motion possible before surgery. The physical therapy sessions on obtaining knee extension first, then flexion, and lastly increasing strength. A previous study 450 of our osteoarthritic patients showed that our pre-operative rehabilitation gave great relief of pain and symptoms, and 76% decided they did not need TKA surgery.[2] Patients who go on to have TKA surgery receive the benefit of improved ROM before surgery, which has been found to correlate with improve ROM after TKA surgery [20]. Our experience with seeing patients for second opinions, and talking with other orthopedic surgeons, is that most surgeons do not know that range of motion can improve in an arthritic knee. Most patients, who are not happy with their TKA after surgery, typically demonstrate joint stiffness and weakness. We believe our patients are an active participant in their rehabilitation process, and it is the responsibility of the physical therapist to educate the patient and the orthopedic surgeon on how physical therapy can benefit their osteoarthritic knee patient; both prior to surgery and post-operatively with an emphasis on keeping patients down, preventing the hemarthrosis, and not getting them up to walk same day of surgery.
KEY POINTS
- Preventing a hemarthrosis after knee surgery is the key to preventing and allowing for easier, predictable rehabilitation that improves range of motion and increases strength.
- It is the total pain management and rehabilitation protocol, of using a CPM machine that provides elevation and gentle motion along with IV Ketorolac, cold/compression, TED hose, and bedrest, that prevents a hemarthrosis post-operatively.
- Our post-operative protocol program has proven effective with ACL reconstruction for preventing ROM deficits, which are associated with higher rates of developing osteoarthritis in the long-term.
- In our TKA patients, our post-operative protocol has been proven to reduce pain scores, reduce narcotic pain medication use, and reduce complications; the percentage of TKA patients with KOOS subjective sub-scores in the normal range ranges from 84-96% at 1 year after surgery.
References
- Beaupre, Lauren A. et al. Exercise Combined with Continuous Passive Motion or Slider Board Therapy Compared with Exercise Only: A Randomized Controlled Trial of Patients Following Total Knee Arthroplasty. Physical Therapy Journal. April 2001, Vol 81, Issue 4; pp 1029-1037.
- Benner RW, Shelbourne KD, Bauman SN, Norris A, Gray T. Knee osteoarthritis: Alternative range of motion treatment. Orthopedic Clinics North Am 2019;50:425-432.
- Brosseau, Lucie et al. Efficacy of Continuous Passive Motion Following Total Knee Arthroplasty: A Metaanalysis. The Journal of Rheumatology 2004; 31:11; pp 2251-2264.
- Chandrasekaran, S., Ariaretnam, S.K., Tsung, J. and Dickison, D. (2009), Early mobilization after total knee replacement reduces the incidence of deep venous thrombosis. ANZ Journal of Surgery, 79: 526-529. doi:10.1111/j.1445-2197.2009.04982.x
- Chen, Boquing MD, PhD et al. Continuous Passive Motion After Total Knee Arthroplasty: A Prospective Study. American Journal of Physical Medicine & Rehabilitation, Sept-Oct 2000, Vol 79(5), pp 421-426.
- Coutts RD, Toth C, Kaita JH: The role of continuous passive motion in the postoperative rehabilitation of the total knee patient. p 126. In Hungerford DS, Krackow KA, Kenna RV (eds): Total knee arthroplasty: a comprehensive approach. Williams & Williams, Baltimore, 1984.
- Flevas DA, Megaloikonomos PD, Dimopoulos L, Mitsiokapa E, Koulouvaris P, Mavrogenis AF. Thromboembolism prophylaxis in orthopaedics: an update. EFORT Open Rev. 2018;3(4):136–148. Published 2018 Apr 27. doi:10.1302/2058-5241.3.170018.
- Fresenius Kabi USA, LLC. Ketorolac tromethamine injection, solution [package insert]. Lake Zurich, IL: Fresenius Kabi USA, LLC; 2013.
- Gillis JC, Brogden RN. Ketorolac: a reappraisal of its pharmacodynamics and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997; 53:139-188.
- Howard, Jennifer s. et al. Continuous Passive Motion, Early Weight Bearing, and Active Motion following Knee Articular Cartilage Repair: Evidence for Clinic Practice. Cartilage June 2010, Vol 1(4), pp 276-286.
- Javier Trujillo-Santos, Emilio Perea-Milla, Alberto Jiménez-Puente, Emilio Sánchez-Cantalejo, Jorge del Toro, Enric Grau, Manuel Monreal, RIETE Investigators. Bed rest or ambulation in the initial treatment of patients with acute deep vein thrombosis or pulmonary embolism: findings from the RIETE registry. Chest. 2005 May; 127(5): 1631–1636. doi: 10.1378/chest.127.5.1631.
- Knapik DM, Harris JD, Pangrazzi G, et al. The basic science of continuous passive motion in promoting knee health: a systematic review of studies in a rabbit model. Arthroscopy. 2013;29(10):1722–1731. doi:10.1016/j.arthro.2013.05.028.
- Lau, Sing Ki Kenric, Chiu, Kwong-Yuen. Use of Continuous Passive Motion After Total Knee Arthroplasy. The Journal of Arthroplasy, 2001; Vol. 16, No. 3; pp 336-339.
- Lenssen, Ton AF et al. Effectiveness of prolonged use of continuous passive motion (CPM), as an adjunct to physiotherapy, after total knee arthroplasty. BMC Musculoskeletal Disorders April 2008, Vol 9(60) pp 1471-2474.
- Liu Z, Tao X, Chen Y, Fan Z, Li Y. Bed rest versus early ambulation with standard anticoagulation in the management of deep vein thrombosis: a meta-analysis. PLoS One. 2015;10(4):e0121388. Published 2015 Apr 10. doi:10.1371/journal.pone.0121388.
- Lynch, AF et al. Deep-vein Thrombosis and Continuous passive Motion after Total Knee Arthroplasty. J Bone Joint Surg Am. 1988; 70: 11-14.
- MacDonald, Steven J, Bourne, Robert B, et al. Clinical Orthopaedics and Related Research. Nov 2000, No. 380; pp 30-35.
- McInnes, Janice MPH et al. A Controlled Evaluation of Continuous passive Motion in Patients Undergoing Total Knee Arthroplasty. JAMA, Sept 1992, Vol 268, No. 11, pp 1423-1428.
- Özcan M, Erem M, Turan FN. Symptomatic Deep Vein Thrombosis Following Elective Knee Arthroscopy Over the Age of 40. Clin Appl Thromb Hemost. 2019;25:1076029619852167. doi:10.1177/1076029619852167.
- Ritter MA, Gandolf VS, Holston KS: Continuous passive motion versus physical therapy in total knee arthroplasty. Clin Orthop 233-239, 1989.
- Salter RB, Simmons Df, Malcom BW: The effects of continuous passive motion on full thickness defects in articulator cartilage: an experimental investigation in the rabbit. J Bone Joint Surg Am 1975; 57A: 570-1.
- Salter RB, Simmonds DF, Malcolm BW, et all: The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage: an experimental investigation in the rabbit. J Bone Joint Surg Am 1980; 62A:1232-51.
- Schwinghammer AJ, Isaacs AN, Benner RW, Freeman H, O’Sullivan JA, Nisly SA. Continuous infusion ketorolac for postoperative analgesia following unilateral total knee arthroplasty. Ann Pharmacother. 2017;51:452-456.
- Shelbourne KD, Liotta FJ, Goodloe SL. Preemptive pain management program for anterior cruciate ligament reconstruction. Am J Knee Surg. 1998; 11:116-119.
- Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990; 18:292-299.
- Shelbourne KD, Benner, RW, Gray T. Results of anterior cruciate ligament reconstruction with patellar tendon autografts. Objective factors associated with the development of osteoarthritis at 20 to 33 years after surgery. Am J Sports Med. 2017; 45:2730-2738.
- Shelbourne KD, Freeman H, Gray T. Osteoarthritis after Anterior Cruciate Ligament Reconstruction: The Importance of Regaining and Maintaining Full Range of Motion. Sports Health. Sports Health. 2012;4(1):79-85.
- Shelbourne, KD, Urch SE, Gray T, Freeman H. Loss of Normal Knee Motion After Anterior Cruciate Ligament Reconstruction is Associated with Radiographic Arthritic Changes After Surgery. Am J Sports Med. 2012;40:108-113.
- Ververeli, Prodromos A., Sutton, Douglas C, et al. Continuous Passive Motion After Total Knee Arthroplasty: Analysis of Cost and Benefits. Clinical Orthopaedis and Related Research. Dec 1995, No. 321; pp 208-215.

